The countless cytotoxic effects of traditional chemotherapy have created the need for novel treatments for cancer. Oncolytic virus therapy (OV) is a gene therapy that combines the benefits of immunotherapy. It is already counting four marketed products, over fifty assets in clinical trials, and many more at the preclinical stage.
The technology behind oncolytic viruses
When administered to any cancer patient, an OV replicates and lyses the malignant cells without inflicting any harm on the healthy ones. Various genetic engineering tools are used to modify the viral DNA/RNA to delete the virulent genes rendering them non-pathogenic; and to incorporate transgenes such as cytokines (GM-CSF, Interferon-γ, TNFα), chemokines (CCL4, CXCL 9, CXCL10, CXCL12), checkpoint inhibitors (PD-1/PDL1, CTLA-4, TIGIT), CAR-T and suicide genes (HSV-TK) making the cell killing stronger and more specific. Low doses of cytotoxic and radioactive drugs can also be carried by the OV to deliver them directly into the tumor.
The most widely used virus types in the development of oncolytic vectors are herpes simplex virus (HSV), adenovirus, and vaccinia. However, reovirus, Newcastle Disease virus (NDV), coxsackie A virus, and rabbit pox virus are becoming increasingly common.
HSV was widely used because of the relatively large, double-stranded, linear DNA genome, which made it easier to alter. Currently, most OV products use oncolytic adenovirus and unmodified reovirus.
Evolving Oncolytic viruses landscape
Below we discuss the approved treatments in different regions and the products already in the pipeline.
Approved products in the oncolytic viruses space
- Amgen‘s IMLYGIC (Talimogene laherparepvec), for the treatment of unresectable melanoma recurrent after initial surgery, was the first oncolytic virus that the US and EU approved in 2015
- Earlier, in 2004, Russia had approved RIGVIR by the Rigvir group for the prevention of relapse and metastases of melanoma after radical surgery.
- And in 2005, China approved ONCORINE by Shanghai Sunway Biotech for treating head and neck cancer.
- Most recently, in 2021, Japan approved DELYTACT for malignant glioma.
Oncolytic Virus Treatments: Notable players in the space
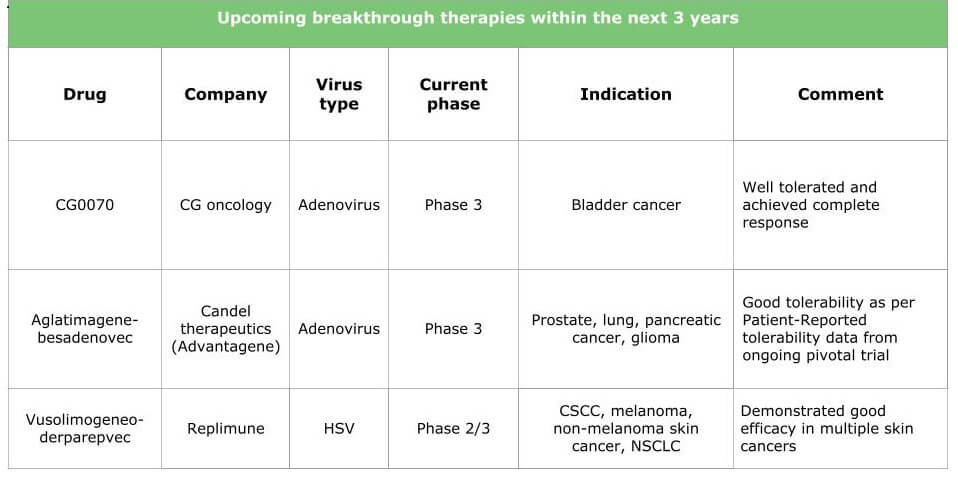
Ongoing research will result in therapies for various types of cancer. The tables below summarize the current situation by presenting the products under clinical development (HSV, adenovirus, vaccinia and other viruses are already in the pipeline) and the breakthrough therapies we expect within the next three years.
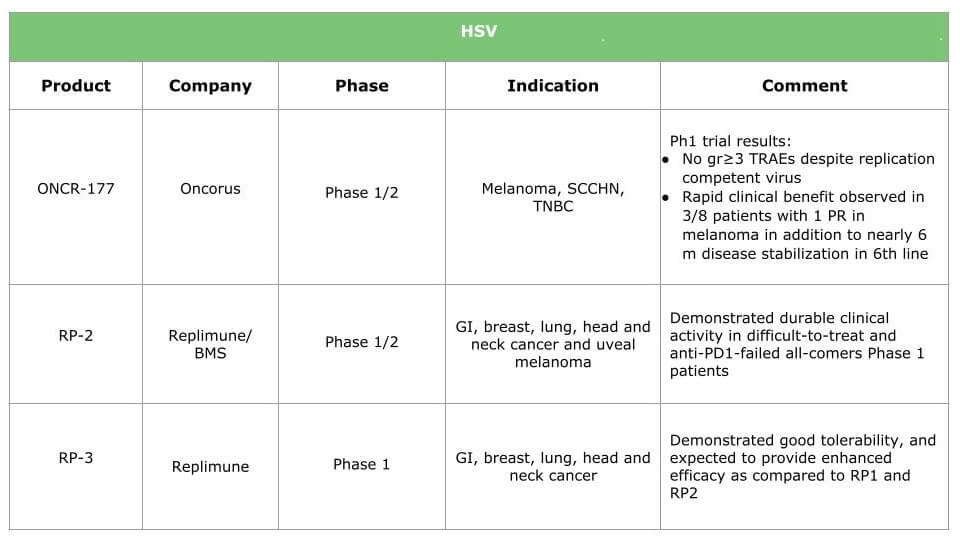
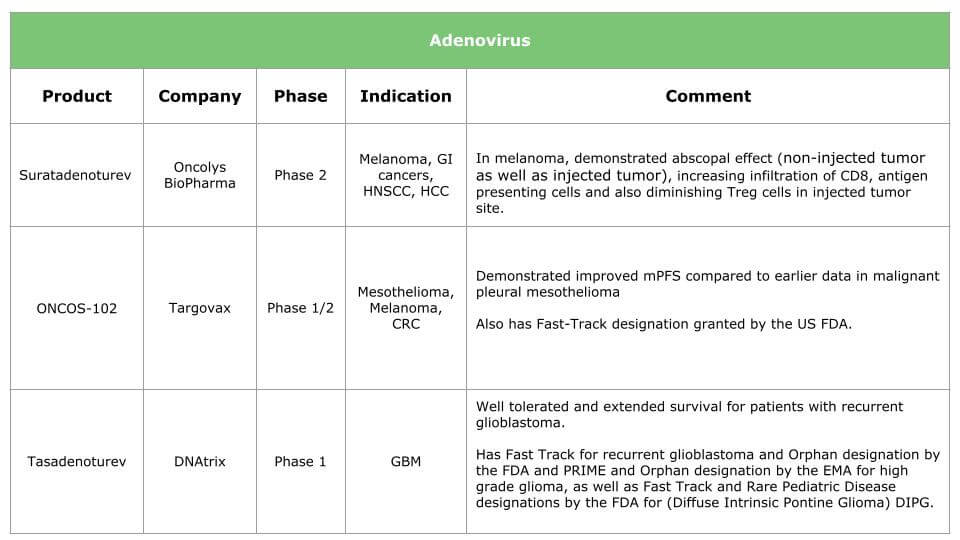
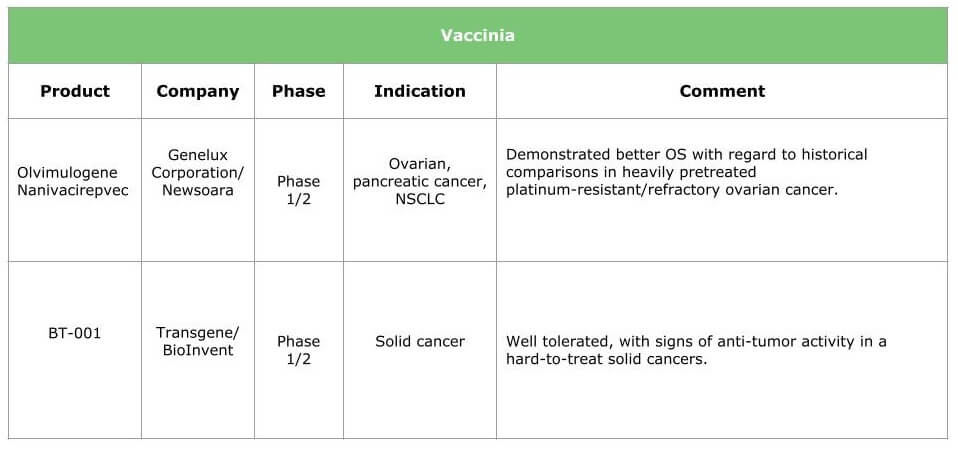
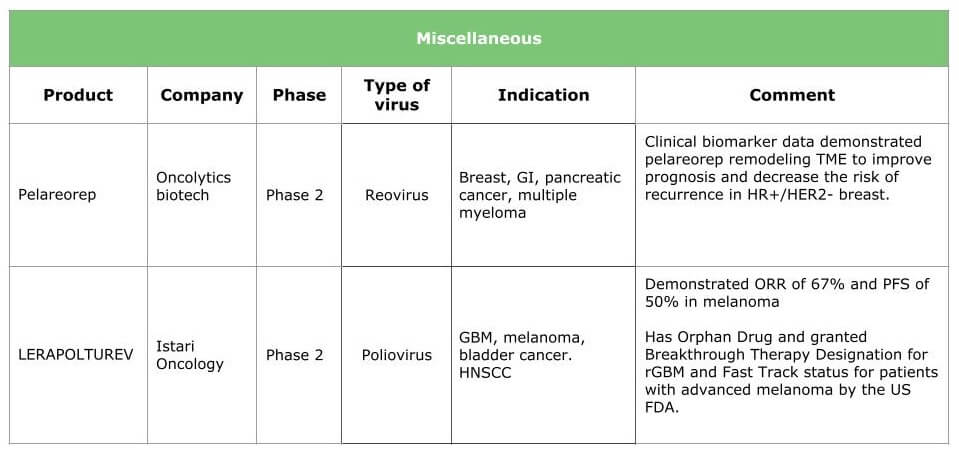
Potential benefits of oncolytic virotherapy
Oncolytic virotherapy brings to the table the following desired features that promise to improve cancer treatment.
- OV targets only the malignant cells without interfering with the healthy cells; hence there is no cytotoxic effect or myelosuppression.
- Arming the OV with oncogenes helps identify resistant tumors and induces cell death where other therapies fail.
- Some oncolytic virotherapy treatments are designed to turn “cold” tumors into “hot” ones, thus activating the immune response (hot tumor: immune system actively produces antibodies against the tumor; cold tumor: the tumor is not “seen” by the immune system)
Challenges of oncolytic virus-based therapies
While this viral therapy addresses certain patients’ unmet needs, it has limitations regarding adverse effects, intratumoral penetration, types of cancer it treats, cost, patient burden, and virulence control.
- Although this viral therapy has fewer adverse effects compared to other types of therapy, immune-mediated reactions are most frequently observed (the immune system views the oncolytic virus as an antigen and produces antibodies against it)
- Another limitation is the relatively poor intratumoral penetration by the oncolytic viruses, which were modified to have a limited replicative potential
- Oncolytic virotherapy can be used against solid cancers, not hematological ones since the viruses cannot replicate in the blood cells.
- Due to heavy genetic engineering, the drug has high costs.
- Self-administration of the drug is not possible; hence patient must visit the hospital for each dose.
- In rare cases, the patient suffers from the virulence of the virus.
Oncolytic virus treatments: Future Prospects and Opportunities
With the discovery of new oncolytic virus vectors, innovative strategies are being used to counter the disadvantages of previous generations of oncolytic viral vectors. Scientists now focus on new concepts for more efficient targeting and explore lower-cost solutions.
For example, researchers continue to work on different engineering techniques to target tumors with certain mutations (PD1, CTLA4)
At the same time, they explore the reduction of manufacturing costs by using vector types that require fewer modifications. For example, unmodified OV (REOLYSIN, Pelareorep) is non-pathogenic to humans and is being used without any genetic modification, thereby reducing overall manufacturing costs.
With the currently approved products in this space, and the significant number of new assets in the pipeline, the future for oncolytic virus treatments for types of resistant and recurrent cancers, including cold tumors, seems bright.
Do you want to stay updated on oncolytic virotherapy and other latest cancer treatment options?
Contact us and gain best-in-class market insights. Learn how we help you make informed decisions.
#oncolyticvirotherapy #cancertreatment #cancerresearch #oncolyticvirus #oncology
Sources:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7589713/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4918352/
https://pubmed.ncbi.nlm.nih.gov/32078405/
https://pubmed.ncbi.nlm.nih.gov/32170083/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7509414/
https://purplebooksearch.fda.gov/results?query=Talimogene%20laherparepvec&title=Imlygic
https://replimune.com/our-science/
https://www.cgoncology.com/technology/#overview
https://www.candeltx.com/pipeline/
https://www.oncolyticsbiotech.com/technology
https://www.oncorus.com/our-technology/
https://www.targovax.com/en/oncos-oncolyctic-virus/
https://www.daiichisankyo.com/files/news/pressrelease/pdf/202111/20211101_E.pdf
https://www.transgene.fr/en/technology/
https://www.genelux.com/overview/
https://www.istarioncology.com/science/lerapoleturev-moa
https://www.dnatrix.com/pipeline/